What Is Supercooling In Chemistry
21-04-2010
Supercooling, a state where liquids do non solidify even below their normal freezing point, withal puzzles scientists today. A good example of this phenomenon is found everyday in meteorology: clouds in high altitude are an aggregating of supercooled droplets of h2o beneath their freezing indicate. Scientists from the Commissariat à l'Energie Atomique et aux Energies Alternatives (CEA), the Heart National de Recherche Scientifique (CNRS) and the ESRF have institute an experimental caption of the phenomenon of supercooling. Their research is published this calendar week in Nature.
Supercooled liquids are trapped in a metastable state even well below their freezing indicate, which tin only be achieved in liquids that practise non comprise seeds that may trigger crystallization. Clouds at high altitude are a good case for this: they incorporate tiny droplets of water that, in the absence of seed crystals do non form ice despite the low temperatures. In everyday life, though, in that location is usually some crystalline impurity in contact with the liquid that volition trigger the crystallization process, and therefore the freezing. Controlling solidification behaviour is of import for applications ranging from hail prevention upward to technological processes such as welding and casting or even the growth of semiconductor nanostructures.
Supercooling was discovered already in 1724 by Fahrenheit, but even today the phenomenon remains a subject for intense discussions. Over the terminal 60 years the very existence of deep supercooling has led to speculations that the internal construction of liquids could be incompatible with crystallization. Models suggest that a significant fraction of the atoms in liquids adjust in v-fold coordinated clusters. To form a crystal however, one needs a structure that can be repeated periodically, filling the entire infinite. This is non possible with five-fold coordinated clusters. In the two-dimensional analogue, a plane cannot be filled past pentagons only, whereas triangles, rectangles or hexagons tin can fill up a aeroplane perfectly. In this example, pentagons are an obstruction to crystallization.
Until today there was no experimental proof that this five-fold coordinated structures are at the origin of supercooling. The researchers from the CEA, CNRS and ESRF studied the structure of a item liquid, a gold-silicon alloy, in contact with a particularly decorated silicon (111) surface, where the outermost layer of the solid featured pentagonal diminutive arrangements. Their findings confirmed that a stiff supercooling outcome took place. "We studied what happened to the liquid in contact with a 5-fold coordinated surface", explains Tobias Schülli, first writer of the paper. The team performed the command experiment with the aforementioned liquid exposed to 3-fold and four-fold coordinated surfaces, which reduced the supercooling event dramatically. "This constitutes the first experimental proof that pentagonal order is at the origin of supercooling", explains Tobias Schülli.
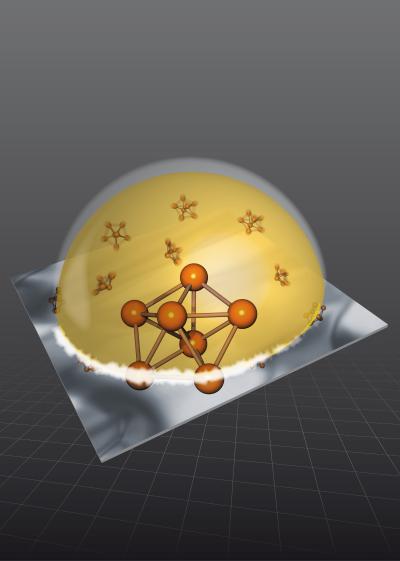 |
| Droplet of a gold-silicon liquid alloy on a silicon (111) surface. Pentagonal clusters formed at the interface exhibit a denser structure compared to solid golden and preclude the liquid from crystallization at temperatures as low as 300 Kelvin beneath the solidification temperature. Graphics: M.Collignon |
It was during their studies, originally focusing on the growth of semiconducting nanowires, that the scientists discovered the unusual backdrop of these liquids. As they were observing the first phase of growth of nanowires, they could see that the metallic-semiconductor alloy they used remained liquid at a much lower temperature than its crystallization signal then they decided to investigate this phenomenon. These liquid alloys are popular in applied research every bit they enable the growth of sophisticated semiconductor nanostructures at low growth temperatures. Most of these nanowire structures are grown on silicon (111), the same surface used by the team. Semiconducting nanowires are promising candidates for futurity electronic devices. Prominent examples are solar cells, where scientists are working on the integration of silicon nanowires in social club to increase their performance. A new instrument for growth studies on silicon nanowire structures is currently being commisssioned at the ESRF, with the fiscal support of the Fondation Nanosciences.
When pure h2o is cooled beneath freezing point, information technology may remain in a supercooled land. It tin can so rapidly crystallize into ice when stimulated past an advisable goad, such every bit shaking the bottle. Credits: J. Cusack.
Reference:
Schülli, T.U. et al, Nature, 22 April 2010.
What Is Supercooling In Chemistry,
Source: https://www.esrf.fr/news/general-old/general-2010/supercooling
Posted by: ochoascang1935.blogspot.com


0 Response to "What Is Supercooling In Chemistry"
Post a Comment